Research Projects
- HOME
- Research Projects
- Role of chromatin modifiers in cerebellar development and medulloblastoma oncogenesis
- Oncogenic mechanisms of brain tumor-specific fusion genes
- Brain tumor microenvironment
- Mechanisms of amyloid accumulation, Aβ catabolism and metabolism in Alzheimer’s disease
Role of chromatin modifiers in cerebellar development and medulloblastoma oncogenesis
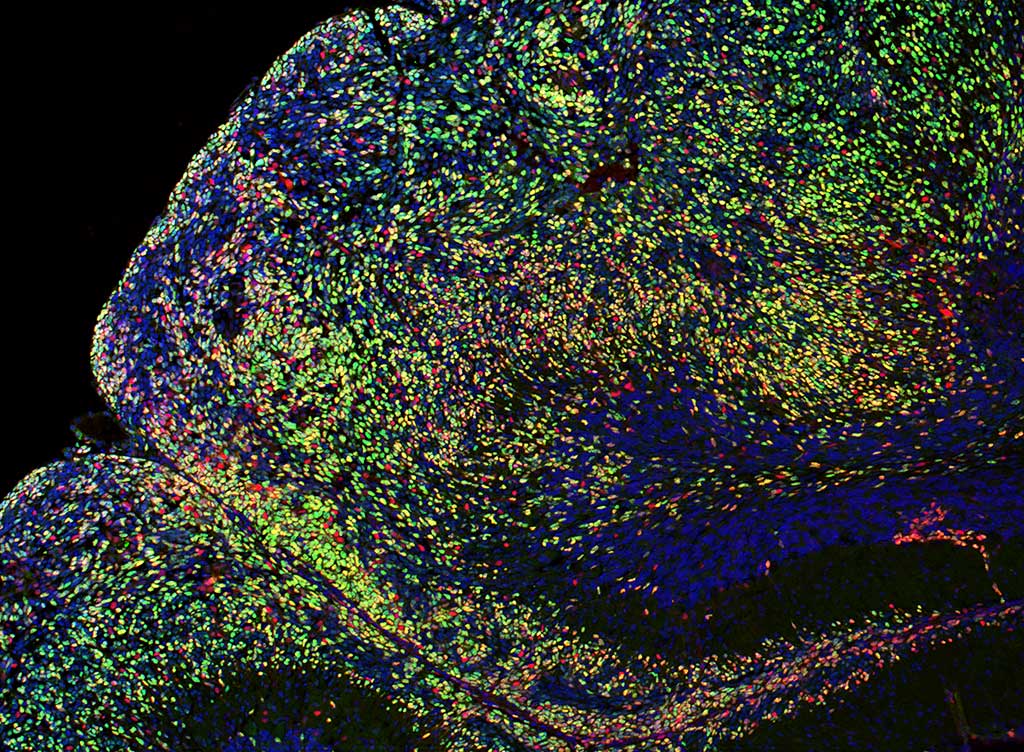
Recent advances in short-read sequencing technology have greatly advanced our understanding of medulloblastoma, a type of pediatric brain tumor, at the molecular level. In particular, mutations in chromatin regulators (proteins that regulate folding and compaction of DNA) are common, and are known to alter the epigenome (the set of markers on the genome governing gene expression).
However, the specific mechanisms by which mutation to chromatin regulators, and the resulting global changes to epigenetic regulation, contribute to development and progression of tumors is not yet well-understood.
Our research seeks to clarify the role of chromatin regulators in normal cerebellar development using mouse models and genome editing technologies such as CRISPR. We aim to understand oncogenesis by examining what epigenome and gene expression changes occur in the resulting tumors when mutations are introduced to chromatin regulators in these mouse models.
By this approach, which focuses not only the cancer blueprint (genome), but also how that blueprint is read (epigenome), we hope to accelerate the development of new therapies for epigenetically driven tumors.
Relevant publications: Zuckermann et al. Nat Commun. 2015; Feng et al. Nat Commun. 2017; Kutcher et al. Genes Dev. 2020; Shiraishi et al. Dev Cell 2024; Wang et al. Cell Rep 2025.
Oncogenic mechanisms of brain tumor-specific fusion genes
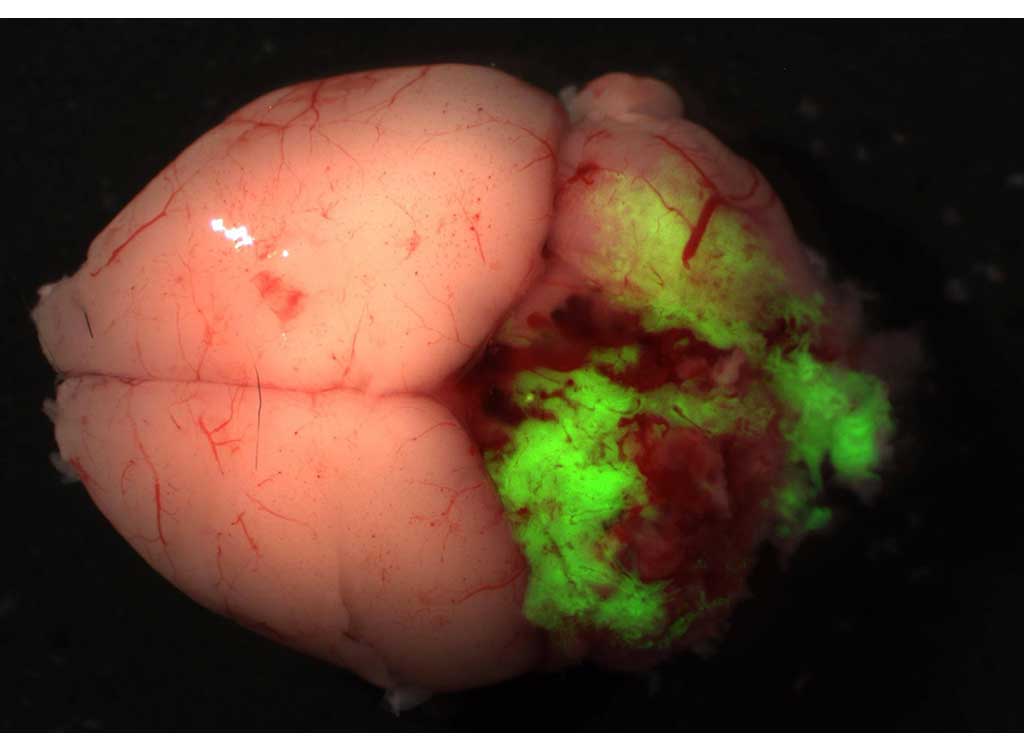
In some cancers, a structural variant, or rearrangement of the genome sequence, can sometimes create a new gene called a fusion gene composed of partial sequences of two normal genes. These fusion genes, which are not found in normal cells, have cancer-specific functions which make them powerful drivers of cancer development and progression.
The importance of fusion genes in cancer research was highlighted by a 2016 “Blue Ribbon Panel” of top cancer researchers in the United States as a top priority in pediatric cancer research.
Our research focuses on common fusion genes in pediatric brain tumors, using in vivo models to determine which cancer-specific signalling pathways they activate.
Our results are reflected in the latest molecular classification of brain tumors by the World Health Organization (WHO), revised in 2021, which defines some ependymoma subgroups by the presence of specific fusion genes. These classifications have important implications for tumor prognosis and treatment strategies. Thus, by understanding the molecular mechanisms of fusion genes in brain tumors, we aim to drive new insights into cancer etiology and develop tumor-specific therapeutic strategies.
Relevant publications: Pajtler et al. Nat Commun. 2019; Zheng et al. Cancer Discov 2021
Brain tumor microenvironment
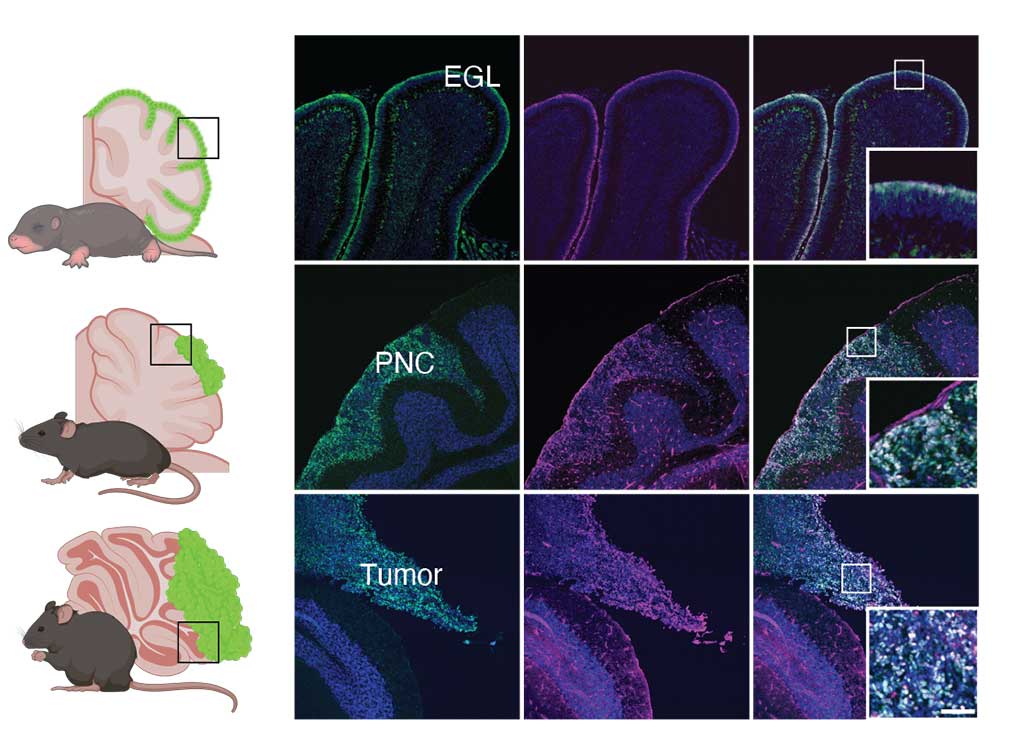
A growing body of research suggests that brain tumor cells can manipulate neighboring neurons and glial cells to create a microenvironment with favorable conditions for cancer growth.
Interactions between tumor and normal brain cells are believed to drive progression and malignancy of cancer. To understand the mechanism, it is important to observe these interactions in vivo.
We have developed a pediatric brain tumor model in our lab to investigate such mechanisms. In addition, we collaborate with Prof. Naofumi Uesaka at Institute of Science Tokyo and Dr. Olivier Ayrault at Institut Curie, France to open interdisciplinary research using omics analysis of mouse models.
This research is expected to drive therapeutic discovery by reconsidering cancer as a living organism interacting with its environment in the brain.
Relevant publications: Forget et al. Cancer Cell 2018
Mechanisms of amyloid accumulation, Aβ catabolism, and Aβ metabolism in Alzheimer’s disease
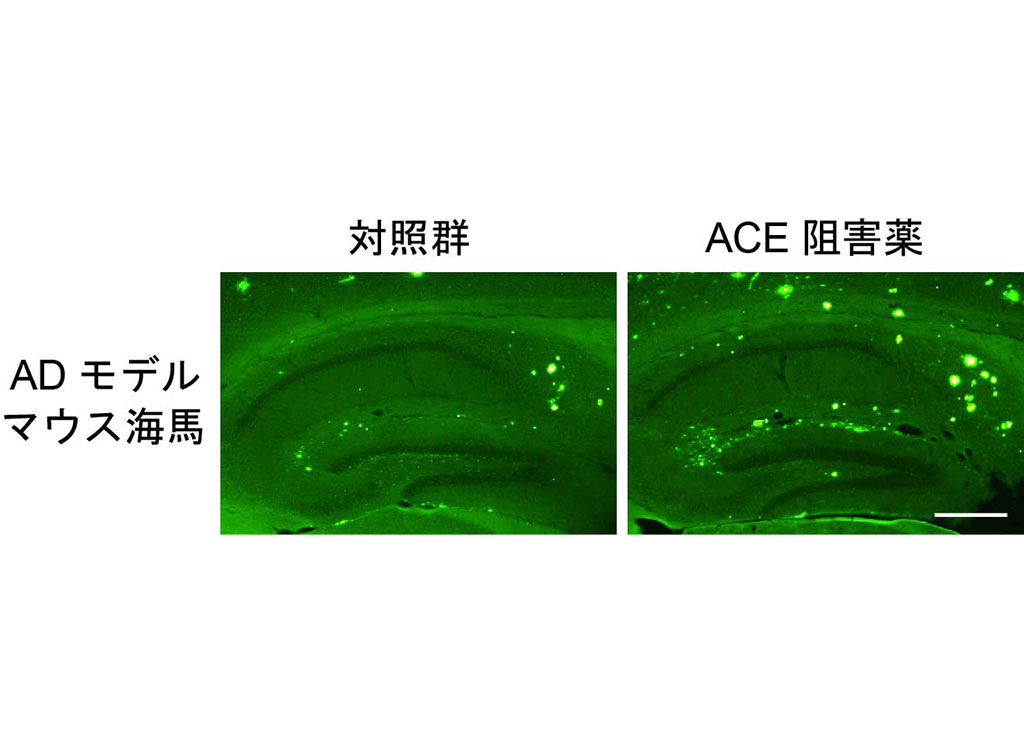
As the Japan’s population ages, the number of dementia patients continues to increase. In particular, there is urgent need to identify methods of prevention and treatment for Alzheimer’s disease, which accounts for an estimtaed 60% of dementia cases. Our team aims to elucidate the pathophysiology of Alzheimer’s disease and other neurodegenerative diseases, and to identify molecular interventions for prevention and treatment.
Accumulation of amyloid-β protein (Aβ) in the brain is widely believed to be deeply involved in the pathogenesis of Alzheimer’s disease. We previously discovered that the Aβ42 peptide is highly neurotoxic, while Aβ40 is neuroprotective. We found that (i) Aβ42 is highly aggregative and therefore neurotoxic, but Aβ40, which can exist in monomeric form, chelates transition metals and suppresses the generation of reactive oxygen species (ROS), inhibiting ROS-induced neuronal cell death; and (ii) Aβ40 binds to Aβ42, inhibiting Aβ42 aggregation into neurotoxic amyloid plaques. As a consequence, monomeric Aβ40 has an inhibitory effect on the neurotoxicity of Aβ42.